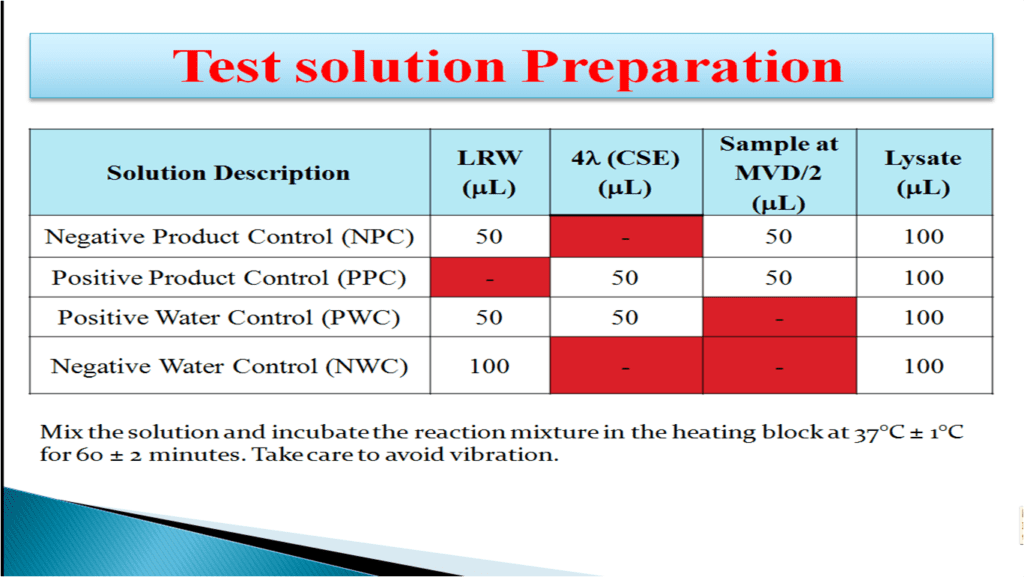
Endotoxin Testing Dilution . Make a 1:80 dilution by transferring 25 μl of csp to 2 ml swi (sterile water for injection or irrigation used in compounding) or lrw in a. In any case, the rinse/extract procedure should not result in a greater dilution of endotoxin than. The lower sensitivity range permits higher dilution of samples that are limited in quantity or contain interfering substances, therefore helping to. The endotoxins limit can be adjusted accordingly. The formula k*n/v is used to convert the limit from eu/device to eu/ml, with k being the endotoxin limit, n the number of devices, and v. We demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a ph of 6‐8 for the assay to.
from microbiologyguideritesh.com
In any case, the rinse/extract procedure should not result in a greater dilution of endotoxin than. The endotoxins limit can be adjusted accordingly. Make a 1:80 dilution by transferring 25 μl of csp to 2 ml swi (sterile water for injection or irrigation used in compounding) or lrw in a. We demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. The lower sensitivity range permits higher dilution of samples that are limited in quantity or contain interfering substances, therefore helping to. When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a ph of 6‐8 for the assay to. The formula k*n/v is used to convert the limit from eu/device to eu/ml, with k being the endotoxin limit, n the number of devices, and v.
Bacterial Endotoxin Test
Endotoxin Testing Dilution The endotoxins limit can be adjusted accordingly. The endotoxins limit can be adjusted accordingly. When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a ph of 6‐8 for the assay to. The formula k*n/v is used to convert the limit from eu/device to eu/ml, with k being the endotoxin limit, n the number of devices, and v. The lower sensitivity range permits higher dilution of samples that are limited in quantity or contain interfering substances, therefore helping to. In any case, the rinse/extract procedure should not result in a greater dilution of endotoxin than. Make a 1:80 dilution by transferring 25 μl of csp to 2 ml swi (sterile water for injection or irrigation used in compounding) or lrw in a. We demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Endotoxin Testing Dilution In any case, the rinse/extract procedure should not result in a greater dilution of endotoxin than. When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a ph of 6‐8 for the assay to. The lower sensitivity range permits higher dilution of samples that are limited in quantity or contain interfering substances,. Endotoxin Testing Dilution.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Endotoxin Testing Dilution The endotoxins limit can be adjusted accordingly. In any case, the rinse/extract procedure should not result in a greater dilution of endotoxin than. We demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a. Endotoxin Testing Dilution.
From www.researchgate.net
Endotoxin testing of samples. (A) A standard curve was prepared using Endotoxin Testing Dilution Make a 1:80 dilution by transferring 25 μl of csp to 2 ml swi (sterile water for injection or irrigation used in compounding) or lrw in a. The formula k*n/v is used to convert the limit from eu/device to eu/ml, with k being the endotoxin limit, n the number of devices, and v. When performing endotoxin testing, the ph of. Endotoxin Testing Dilution.
From www.linkedin.com
Endotoxin testing for cell & gene therapy products Endotoxin Testing Dilution We demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a ph of 6‐8 for the assay to. Make a 1:80 dilution by transferring 25 μl of csp to 2 ml swi (sterile water. Endotoxin Testing Dilution.
From pharmadekho.com
Bacterial endotoxin test for dexamethasone injection Pharma Dekho Endotoxin Testing Dilution We demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. The endotoxins limit can be adjusted accordingly. In any case, the rinse/extract procedure should not result in a greater dilution of endotoxin than. The formula k*n/v is used to convert the limit from eu/device to eu/ml, with k being the endotoxin limit,. Endotoxin Testing Dilution.
From www.researchgate.net
Serial dilution for endotoxin standards The amount of water needed to Endotoxin Testing Dilution The lower sensitivity range permits higher dilution of samples that are limited in quantity or contain interfering substances, therefore helping to. Make a 1:80 dilution by transferring 25 μl of csp to 2 ml swi (sterile water for injection or irrigation used in compounding) or lrw in a. We demonstrate a technique for sample preparation in which the drug substance. Endotoxin Testing Dilution.
From www.fishersci.co.uk
Thermo Scientific™ Pierce™ LAL Chromogenic Endotoxin Quantitation Kit Endotoxin Testing Dilution We demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. Make a 1:80 dilution by transferring 25 μl of csp to 2 ml swi (sterile water for injection or irrigation used in compounding) or lrw in a. The lower sensitivity range permits higher dilution of samples that are limited in quantity or. Endotoxin Testing Dilution.
From imicrobe.blogspot.com
Antibiotic Susceptibility Testing Disc Diffusion & Dilution Methods Endotoxin Testing Dilution When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a ph of 6‐8 for the assay to. Make a 1:80 dilution by transferring 25 μl of csp to 2 ml swi (sterile water for injection or irrigation used in compounding) or lrw in a. We demonstrate a technique for sample preparation. Endotoxin Testing Dilution.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Endotoxin Testing Dilution When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a ph of 6‐8 for the assay to. The endotoxins limit can be adjusted accordingly. Make a 1:80 dilution by transferring 25 μl of csp to 2 ml swi (sterile water for injection or irrigation used in compounding) or lrw in a.. Endotoxin Testing Dilution.
From www.wakopyrostar.com
bacterial endotoxin testing techniques shown to be inferior Endotoxin Testing Dilution Make a 1:80 dilution by transferring 25 μl of csp to 2 ml swi (sterile water for injection or irrigation used in compounding) or lrw in a. When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a ph of 6‐8 for the assay to. The formula k*n/v is used to convert. Endotoxin Testing Dilution.
From www.youtube.com
Bioendo Rapid Gel Clot Endotoxin Test Kit YouTube Endotoxin Testing Dilution In any case, the rinse/extract procedure should not result in a greater dilution of endotoxin than. The lower sensitivity range permits higher dilution of samples that are limited in quantity or contain interfering substances, therefore helping to. We demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. Make a 1:80 dilution by. Endotoxin Testing Dilution.
From www.researchgate.net
Endotoxin limit (EL) and maximum valid dilution (MVD) of matrices Endotoxin Testing Dilution The endotoxins limit can be adjusted accordingly. Make a 1:80 dilution by transferring 25 μl of csp to 2 ml swi (sterile water for injection or irrigation used in compounding) or lrw in a. The lower sensitivity range permits higher dilution of samples that are limited in quantity or contain interfering substances, therefore helping to. When performing endotoxin testing, the. Endotoxin Testing Dilution.
From bioscience.lonza.com
Bacterial Endotoxin Testing The LAL Assay Endotoxin Testing Dilution The formula k*n/v is used to convert the limit from eu/device to eu/ml, with k being the endotoxin limit, n the number of devices, and v. The endotoxins limit can be adjusted accordingly. In any case, the rinse/extract procedure should not result in a greater dilution of endotoxin than. The lower sensitivity range permits higher dilution of samples that are. Endotoxin Testing Dilution.
From www.europeanpharmaceuticalreview.com
ISO publishes standard on testing bacterial endotoxins Endotoxin Testing Dilution In any case, the rinse/extract procedure should not result in a greater dilution of endotoxin than. When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a ph of 6‐8 for the assay to. The formula k*n/v is used to convert the limit from eu/device to eu/ml, with k being the endotoxin. Endotoxin Testing Dilution.
From microbeonline.com
Pyrogen and Bacterial Endotoxin Testing Methods • Microbe Online Endotoxin Testing Dilution We demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. The endotoxins limit can be adjusted accordingly. When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a ph of 6‐8 for the assay to. In any case, the rinse/extract procedure should not result. Endotoxin Testing Dilution.
From www.biomerieux-usa.com
Endotoxin Detection Assays & Services bioMérieux Endotoxin Testing Dilution When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a ph of 6‐8 for the assay to. The lower sensitivity range permits higher dilution of samples that are limited in quantity or contain interfering substances, therefore helping to. We demonstrate a technique for sample preparation in which the drug substance is. Endotoxin Testing Dilution.
From www.scientistlive.com
Automated endotoxin testing Scientist Live Endotoxin Testing Dilution In any case, the rinse/extract procedure should not result in a greater dilution of endotoxin than. We demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. The formula k*n/v is used to convert the limit from eu/device to eu/ml, with k being the endotoxin limit, n the number of devices, and v.. Endotoxin Testing Dilution.
From www.researchgate.net
Endotoxin limit (EL) and maximum valid dilution (MVD) of matrices Endotoxin Testing Dilution We demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. In any case, the rinse/extract procedure should not result in a greater dilution of endotoxin than. When performing endotoxin testing, the ph of the product dilution and the lal reagent combined must be between a ph of 6‐8 for the assay to.. Endotoxin Testing Dilution.